QCMud
Sample Matrix Spike


FAQs
Q1) I obtained a low recovery from a sample that I spiked with EasySeed™. Could this be due to the EasySeed™ containing less Cryptosporidium or Giardia than is stated on the Certificate of Analysis?
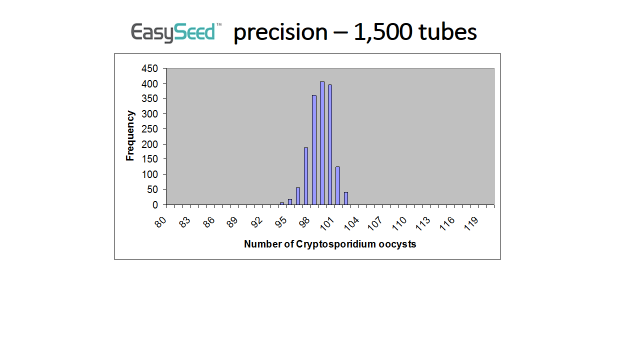
It is extremely unlikely that an EasySeed™ tubes could contain a significantly lower number of Cryptosporidium or Giardia than the number stated on the Certificate of Analysis. Every batch of EasySeed™ is quality controlled by analysing randomly selected tubes. The number of Cryptosporidium and Giardia within each of these tubes is carefully counted. BioPoint has produced over 250 different batches of EasySeed™ and during the quality control a count of lower than 93 Cryptosporidium or Giardia has never been detected. Statistical analysis of this combined quality control data reveals that the chance of an EasySeed™ tube containing less than 92 Cryptosporidium or Giardia is greater than 1 in 1.6 million.
The graph below shows the frequency of counts obtained from analysing EasySeed™.
Low recoveries do occur even in the best testing laboratories in the world. There are many stages in the testing process where something can go wrong that could affect the recovery. For more information please see our Product Note: Tips on improving recoveries in Cryptosporidium and Giardia testing.
Q2) I obtained a recovery of much more than 100% from a sample that I spiked with EasySeed™. Could this be due to the EasySeed™ containing more Cryptosporidium or Giardia than is stated on the Certificate of Analysis?
It is extremely unlikely that an EasySeed™ tube could contain a significantly higher number of Cryptosporidium or Giardia than the number stated on the Certificate of Analysis. As stated in Answer 1 every batch of EasySeed™ is carefully quality controlled. A count of higher than 105 Cryptosporidium or Giardia has never been detected. Statistical analysis of this combined quality control data reveals that the chance of an EasySeed™ tube containing more than 106 Cryptosporidium or Giardia is 1 in 1.6 million!
The most likely cause of obtaining a recovery higher than 100% from a sample spiked with EasySeed™ is cross contamination. The sample has become contaminated with Cryptosporidium or Giardia from another source. Likely sources of contamination include:
- Positive control samples that contain very high numbers of Cryptosporidium and Giardia. We strongly recommend not to work with or store suspensions of Cryptosporidium and Giardia that contain greater than 10,000 Cryptosporidium and Giardia per ml, in the same laboratory that is being used for testing water samples.
- Environment samples such as sewage samples that contain high numbers of Cryptosporidium and Giardia.
For further information see our Product Note: Tips for reducing the risk of cross contamination in C&G testing.
Q3) The morphology or the DAPI fluorescence of the Cryptosporidium and Giardia in samples that have been seeded with EasySeed™ are not as good as the positive control in the EasyStain™ kit. Why is this?
The Cryptosporidium and Giardia can become damaged during the sample processing, particularly the acid dissociation in combination with drying the sample onto the slide. The positive control from the EasyStain™ kit does not go through the IMS and acid dissociation, therefore it is likely to appear less damaged. Tips on how to minimise the effects of the slide drying and acid dissociation on Cryptosporidium and Giardia staining can be found in the EasyStain™ FAQ.
Q4) Can EasySeed™ be used as source of DNA for PCR assays?
The dose of gamma radiation that is used to inactivate the cysts and oocysts in EasySeed™ is small and is unlikely to damage the DNA sufficiently to interfere with PCR analysis.The effect if any would also depend on the target of interest. The radiation is unlikely to affect results for a multicopy target. But If the PCR is detecting a single copy target or a labile target such as rRNA or mRNA then it is probably likely that EasySeed™ may behave differently to fresh cysts and oocysts. We have no data on how stable the DNA is within the oocysts and cysts once they have been irradiated.
We do not have enough information or experience with the actual use of these products in PCR assays to provide more detailed information.
